A team of physicists from the University of Amsterdam has achieved a significant breakthrough in measuring the properties of strontium atoms, a critical element for advanced technologies such as atomic clocks and quantum computers. Their findings, published on November 4, 2025, in the journal Physical Review Letters, detail a novel approach that utilizes rubidium atoms to enhance measurement precision to unprecedented levels.
Strontium, while not widely recognized outside scientific circles, is highly regarded among physicists for its unique properties. As one of the six alkaline earth metals, it possesses 38 protons and various isotopes, with 87 Sr being particularly noteworthy. This isotope’s odd number of nucleons—comprising 38 protons and 49 neutrons—renders it a fermion, which allows it to exhibit unique magnetic properties due to its nuclear spin. This spin is crucial for numerous applications, including the development of highly accurate atomic clocks.
Atomic clocks, which measure time with astonishing precision, rely on the frequencies of light emitted by atoms. For strontium, the optimal optical frequency is associated with a specific red light wavelength of 698 nanometers. The challenge lies in the bosonic isotopes of strontium, which lack the necessary nuclear spin for the ideal atomic transitions. The fermionic nature of 87 Sr allows for the necessary adjustments, enabling effective absorption and emission at stable frequencies.
The work undertaken by the Amsterdam team builds on the principles established by Pieter Zeeman, who discovered the Zeeman effect in 1896. This phenomenon describes how magnetic fields cause the splitting of energy levels within an atom, leading to distinct light frequencies. The accurate measurement of the g-factor—an essential parameter related to the strength of the nuclear magnet—is vital for refining the functionality of optical clocks.
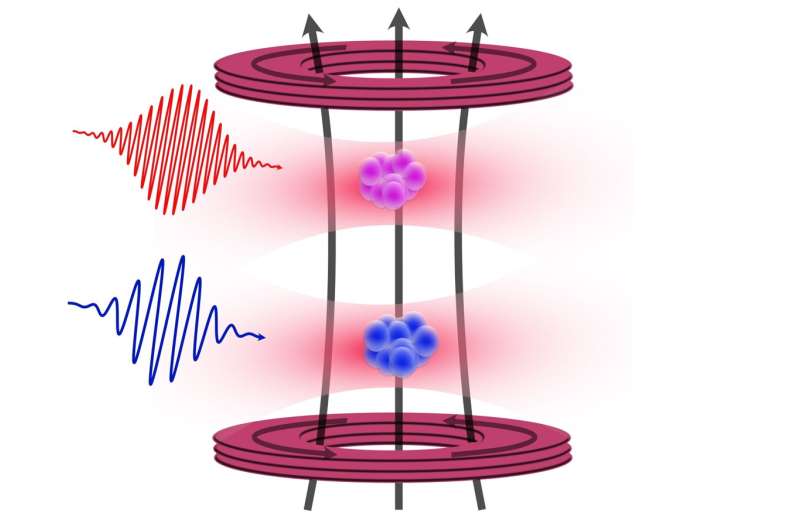
The research team, led by first author Premjith Thekkeppatt, initially aimed to create rubidium-strontium molecules. However, they found that trapping both strontium and rubidium atoms in close proximity, without overlapping, allowed them to utilize a technique called nuclear magnetic resonance. This method enabled precise measurements of the g-factor, leveraging the well-established properties of rubidium to calibrate the magnetic field strength accurately.
This recent advancement represents a hundredfold improvement over previous measurements, setting a new benchmark for atomic structure calculations. Thekkeppatt noted that their findings not only enhance the precision of strontium-based applications but also pave the way for the exploration of other atomic species and their potential uses in various fields.
The implications of this research extend beyond atomic clocks. The ten energy levels that arise from the Zeeman effect in 87 Sr could serve as foundational elements for quantum computing. Unlike classical computers that use bits, quantum computers utilize qubits, which can exist in multiple states simultaneously. The potential to use qudits—analogous to qubits but capable of occupying more than two states—opens up exciting possibilities for future computational power.
As the field of quantum technology continues to evolve, the enhanced measurement techniques developed by the University of Amsterdam team may inspire further breakthroughs, contributing significantly to advancements in both quantum computing and precision measurement. The ongoing exploration of strontium’s properties is likely to yield further insights and applications in the years to come.